What is 2-DG? Do we now have a COVID game changer? How does it work?
Well, on Monday, Defence Minister Rajnath Singh and Health Minister Dr Harsh Vardhan released the first batch of the indigenously developed anti-Covid-19 drug, 2-deoxy-D-glucose or ‘2-DG’. Here’s a good news! This anti-COVID drug can be used as an adjunct therapy in moderate to severe Covid-19 patients. The national drug regulator, Drugs Controller General of India (DCGI), had cleared the formulation on May 1 for emergency use. Scroll down for more details.
Is 2-deoxy-D-glucose or ‘2-DG’ a game changer?
According to the reports, the anti-COVID drug will be administered in select hospitals and June onwards, it can be distributed to all hospitals to be used for treatment of moderate to severe Covid-19 patients.
Who developed this anti-COVID drug?
2-DG, the indigenous drug, has been developed by the Institute of Nuclear Medicine and Allied Sciences (INMAS), New Delhi, a lab of the Defence Research and Development Organisation (DRDO), in collaboration with Hyderabad-based pharma company Dr Reddy’s Laboratories (DRL), the Ministry of Defence had said in a release earlier this month.
How does 2-DG work?
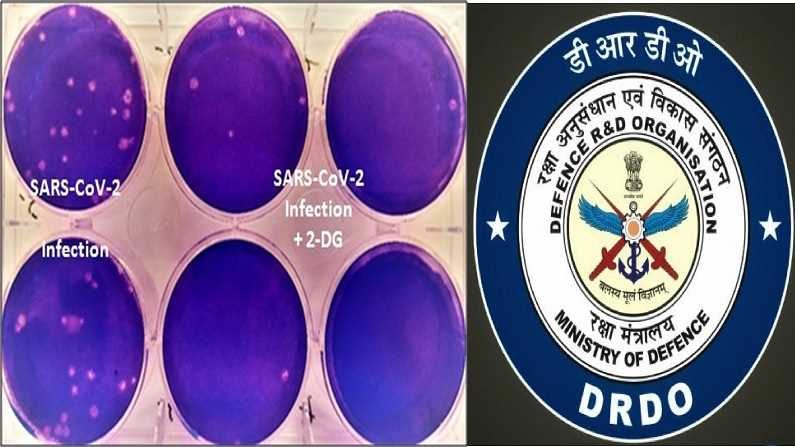
The government has cited that the molecule used in the drug helps Covid-19 affected patients recover faster and their dependency on supplemental oxygen is also reduced. This is definitely a plus point! Besides, as per the reports, the government has also claimed that 2-DG is expected to bring “immense benefit” to the affected patients. The drug, according to a report by The IE, accumulates in virus-infected cells of the body and prevents the virus growth. Because of this, viral synthesis as well as the energy production of the virus can be stopped. The property of only accumulating in virally-infected cells has made this drug unique.
Will 2-DG be a game changer in treating COVID?
Well, looks like it as the clinical trials of the drug started last year in April 2020. The scientists carried out laboratory experiments showing that the molecule is working effectively against the virus- SARS-CoV-2, inhibiting its growth. The phase two clinical trials for this drug was permitted in May 2020 by the Central Drugs Standard Control Organization (CDSCO) in Covid-19 patients.
The report said that the trials were conducted on 110 patients between May and October last year. This was done across six hospitals. After the success of the second phase, DCGI gave permission for phase 3 clinical trials in November 2020. The third phase of clinical trials was concluded in March 2021 where 220 patients were monitored across 27 Covid hospitals.
Data from the phase 3 clinical trial showed that in the 2-DG arm, a “significantly higher proportion of patients improved symptomatically and became free from supplemental oxygen dependence (42% vs 31%) by Day 3 in comparison to SoC, indicating an early relief from oxygen therapy/dependence”, IE quoted the government’s release.
Interestingly, a similar trend was observed in patients aged more than 65 years.
Benefits of 2-DG
The government has said that 2-DG, being a generic molecule and an analogue of glucose, can be easily produced and made available in large quantities. The anti-COVID drug is available in powder form in a sachet, and can be taken orally after dissolving in water.
